Products – Assays
STRATIFYER Molecular Pathology GmbH has developed numerous real time RT-PCR based assays that are compatible with readily available, formalin-fixed paraffin embedded (FFPE) tissues (core needle biopsies, excision biopsies, and surgical samples).
ProliferationTYPER
Quantitative, mulriparametric mRNA assessment of established proliferation markers for exact determination of proliferative activities. In hormone receptor positive, Her-2/neu negative breast cancer the proliferation has been proven to be the strongest prognostic motif and allow to distinguish Luminal A from Luminal B tumors for chemotherapy decision. In neuroendocrine bronchopulmonary tumors the proliferation distinguishes atypical neuroendocrine tumors from small cell lung cancer for surgery decisions.
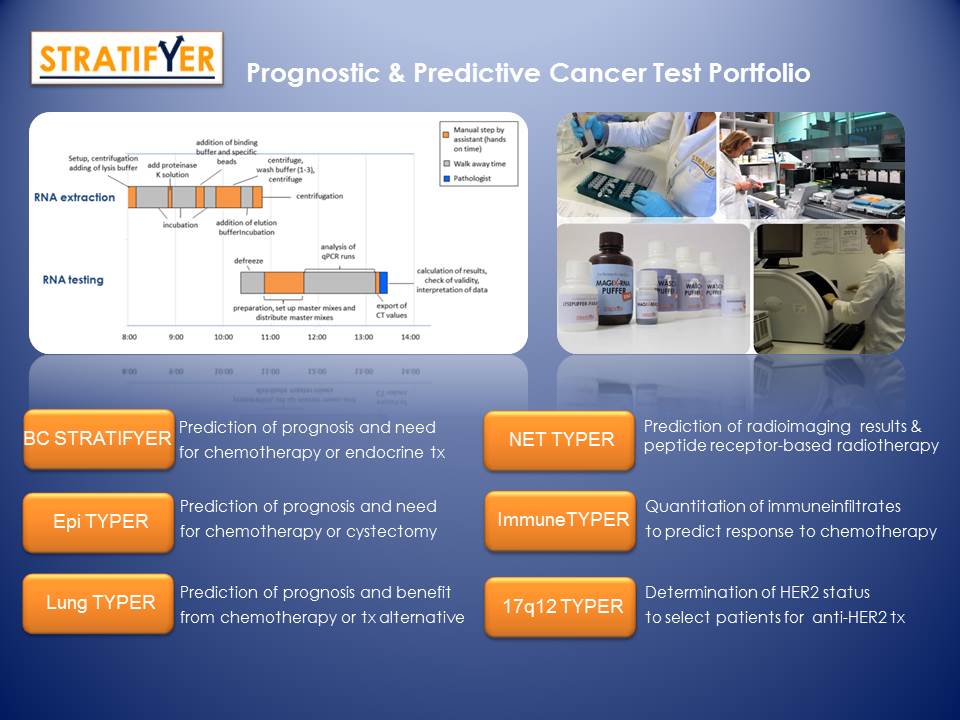
BC STRATIFYER
Quantitative mRNA assessment of the hormone receptors (ESR1, PGR, AR) as well as ERBB2 and proliferation markers (MKI67, RACGAP1) for most accurate assessment of established markers being critical for individual risk assessment, molecular subtyping and targeted treatment decisions in diverse cancer indications including breast and bladder cancer.
Immune TYPER
Quantitative mRNA assessment of immune cell composition in tumor tissues to evaluate the amount of tumor infiltrating B-cells, T-cells, macrophages and their cellular subtypes to determine recurrence risk and potential benefit from chemotherapy.
CheckPoint TYPER
Quantitative mRNA assessment of immunologic target genes to decipher tumor infiltrating immune cell functions and determine potential benefit from immunetherapies.
Epi TYPER
Determination of luminal and non luminal carcinoma by quantitative assesment of differential keratin expression for improved risk assessment upfront of local systemic therapies.
NET TYPER
Determine neuroendocrine subtype for improved risk assessment and quantify theranostic targets for therapeutic treatment decisions.
Lung TYPER
Confirm lung cancer type and determine target genes for alternative treatment options apart from genomics driven mutation targeting.
17q12 TYPER
Assess alterations on chromosome 17q12 including Her-2/neu and neighboring target genes on RNA and DNA level to determine amount of co-amplified genes and potential response to alternative treatment options.
MutationTYPER
Precise analysis of treatment targets (e.g. kRAS, BRAF, ALK) by quantitative PCR technologies on DNA and/or RNA level to stratify for targeted treatment options. The mRNA assessment offers advantages with regard to sensitivity of mutation detection and has been validated in proficiency testings.
Further information are available at info@STRATIFYER.de.